The Political Fix: Why do we know so little about the calculations behind India's vaccine rollout?
Welcome to The Political Fix by Rohan Venkataramakrishnan, a newsletter on Indian politics and policy. To get it in your inbox every week, sign up here.
We don’t charge for this newsletter, but if you would like to support us consider contributing to the Scroll Reporting Fund or, if you’re not in India, subscribing to Scroll+.
The Big Story: Dose over
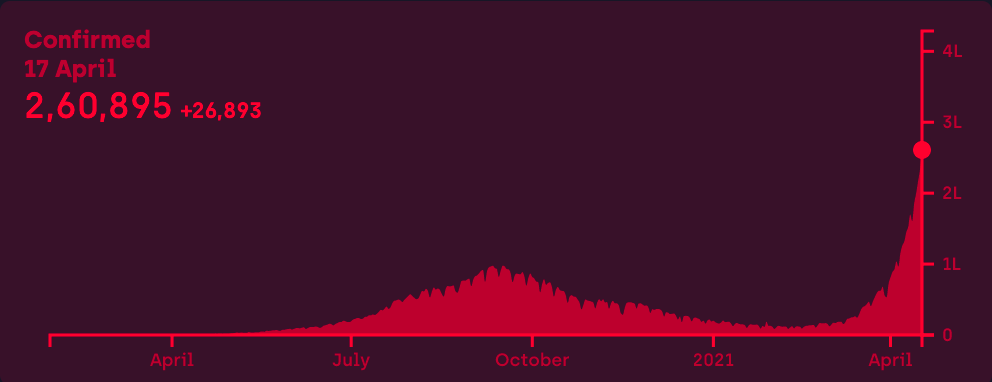
At the height of the Covid-19 crisis in New York in 2020, residents of the city spoke of hearing ambulances go by every few minutes, a sign of just how bad things were. In India, most people on Twitter can attest to something similar over the last week, as the country’s second wave – which we wrote about three weeks ago – appeared to explode.
The social network has been filled with desperate pleas for help, whether in the form of a vacant bed at a Covid-19 hospital or for supplies of oxygen and remdesivir, an anti-viral drug in great demand. Twitter is obviously not representative of the Indian population, but its skew – richer and more privileged – and the sheer volume of demands for help only reflect how bad things are on the ground.




Sitting alongside these requests on the social media feeds are visuals of ambulances lined up outside hospitals, bodies piling up at the gates of crematoriums and long lines of patients queuing up to get medicines.
(There are also many tweets of people aggregating available resources for those in need across the country. You can find some of those collected here and here.)




Unfortunately, these stories aren’t going anywhere. The virus appears to be operating a little differently, putting more younger people in the hospital and spreading much faster than before. We have little understanding of the causes of the second wave, in part because India did not put significant resources into researching virus variants.
After the pain caused in 2020, governments have been reluctant to go back into lockdown or even work to limit large gatherings at election rallies in West Bengal or at the Kumbh Mela in Uttarakhand.
Here is some of our coverage of the Coronavirus crisis over the last week:
Vijayta Lalwani looked at how India’s second wave of Covid-19 is different from the first: faster spread, more younger, i.e. 15-45-year-old patients and even infected children, some doubts about whether RT-PCRs are working well.
Arunabh Saikia reported on how the government sought to downplay Indian variants of the disease – despite warning signs from rural Maharashtra – with the stated aim of avoiding panic, that now seems to have backfired.
Lalwani and Saikia also conducted this investigation into the government’s plans for 150 oxygen generation plants to support hospitals, costing just Rs 200 crore. They found that the government took 8 months just to invite bids, and most aren’t up and running today.
Saikia wrote about why the government seemed unwilling to limit participation at the Kumbh Mela, where millions gathered on the banks of the Ganga in Uttarakhand, until the last few days – because of the Bharatiya Janata Party’s political calculations.
Aarefa Johari reported on a fresh exodus of migrant workers, and limited efforts by state governments despite last year’s example.
Smitha Nair spoke to Dr. Lancelot Pinto, consultant pulmonologist at Mumbai’s PD Hinduja Hospital about why there is so much demand for remdesivir and plasma therapy, despite evidence suggesting they won’t be useful to most.
I wrote about how Modi’s adept communication skills were clearly not being used to convey that India is in a crisis, most likely because it would dent his party’s opportunity to win in the ongoing West Bengal elections.
Help our small team tackle this massive story. Help us go further and dig deeper to bring you insights from across India.
Contribute to the Scroll.in Ground Reporting Fund.
There are lots of elements to India’s second wave story, including baffling ones like why the country still seems stuck on meaningless hygiene theatre and worrisome ones like when will this surge hit its peak, tackled in this thread here.
One of those elements is India’s vaccination campaign.
India has had one of the world’s biggest vaccination rollouts, with more than 3 million doses administered per day through much of April, putting it above the United States and the European Union, both far richer entities. More than 100 million people have received at least one dose – equivalent to a third of the US population.
Given the country’s massive size though, those numbers account for less than 10% of Indians. And that only covers one dose.
If those numbers were not worrying enough against the backdrop of the huge second wave, India’s vaccination campaign – which should have been progressively scaling up taking advantage of the country’s huge vaccine manufacturing base – has actually been losing steam.
I wrote earlier about how, when states began to complain about vaccine shortages, the government decided to resort to an extremely political tirade from the Union Health Minister, in the hopes of blunting any criticism. The government appeared vulnerable to the charge that it had exported more vaccines than administered on the population, something it boasted about earlier in the year.
This has led to calls from Opposition politicians from around the country for the government to halt exports and vaccinate Indians first. Those demands have been criticised by the All India People’s Science Network since it would mean depriving many developing nations of vaccines and also doesn’t take into account how much India has benefited from global arrangements.
Nevertheless, India has severely cut down its vaccine exports, sending just 1.2 million doses abroad in April compared to 64 million between January and March, a move that is worrying many other nations.
It also suddenly gave emergency approval to foreign vaccines that had been cleared for use in a number of other countries, after dragging its feet on the matter for months. This came days after Union Minister Ravi Shankar Prasad labeled former Congress President Rahul Gandhi a “lobbyist” for demanding approvals to foreign vaccines, suggesting at the least that the government’s actions were evidently last-minute.
Here are just a few more questions about India’s vaccination strategy.
What was the game plan?
Union Health Minister Harsh Vardhan initially said that the aim was to fully vaccinate 300 million by August, a target that was later raised to 400 million. Yet it has become evident for some time now that India’s two vaccine manufacturers – the Serum Institute of India and Bharat Biotech – simply do not have the capacity to provide the 800 million doses needed to get to that target.
As Thomas Abraham writes, “the government’s strategy to make up the missing numbers was also never fully disclosed... Perhaps it was thought that demand would be so small that it would be possible to manage with existing capacity. Now, even with the government clamping down on exports, the numbers still do not add up.”
Does the government have a specific blueprint for how many doses it will receive from each manufacturer to make up the August target and beyond?
Where are the orders?
As many have written, the Indian government chose not to take the American route in directly supporting the Serum Institute of India, the world’s largest vaccine manufacturer, as it began to manufacture vaccine doses. Instead, there appeared to be an implicit understanding that half of SII’s vaccines would go to the Indian government, while New Delhi would allow the company to export doses and do deals with other nations.
Even in January 2021, India officially only ordered 11 million doses from SII, followed by another one for 100 million doses in March. “Even now, the government only makes ad-hoc purchases from SII instead of agreeing [to] a longer-term supply schedule,” Reuters reported.
Why hasn’t the government planned ahead on purchase orders? Did its assumptions about exports and domestic use get tripped up by the second wave? Will it articulate its plan going forward?
What is happening with SII and raw materials?
Adar Poonawalla, the CEO of SII, said at the start of April that the company needs Rs 3000 crore to increase its capacity. Two weeks later, this request has yet to be addressed, as made clear by Union Home Minister Amit Shah, who had a caustic response to a question about the demand in an interview.
Per reporting, there are voices within the government concerned about supporting a private firm in this manner. But surely the government could have found another approach to resolving this urgent matter by now?
Even more baffling was a tweet from Poonawalla this week directly to US President Joe Biden, asking him to lift an embargo on raw material exports, which are obstructing Indian vaccine production. Raw material shortages are indeed hurting both SII and Bharat Biotech’s manufacturing plans.



But it seems odd for this to be happening on Twitter, rather than directly or through diplomatic channels. Why is this not an issue that the Indian government has taken up directly with the US, which it is otherwise cooperating on as part of a Quad vaccine manufacturing plan?
Indeed, a related question is this: Why hasn’t India made a bigger noise in its request to the US and other countries to back its demands at the World Trade Organisation for a waiver on vaccine intellectual property rights until the pandemic has ended?
If you haven’t heard about this issue, which has been called a “catastrophic moral failure” of rich nations stockpiling vaccines and private companies sitting on publicly funded vaccine research, read this.
What was the Bharat Biotech plan?
Bharat Biotech’s Covaxin has been touted as a truly Make-in-India success story, since it was jointly developed by the Indian Council for Medical Research and the National Institute of Virology.
But that pedigree should also have meant that the Indian government, which has called unsuccessfully for other countries around the world to waive the licenses of vaccines until the pandemic is over, would have a number of options to produce Covaxin, given that Bharat Biotech’s own capacities still amount to around 12 million doses per month.
Yet the government waited till this week, based on a demand from Maharashtra, to permit the state-owned Haffkine Biopharmaceutical Corporation to manufacture Covaxin. Haffkine’s CEO now says it won’t be able to supply doses until early 2022.
Given that the government knew Bharat Biotech’s manufacturing capacity all along, why did this approval only come as the second wave saw numbers go through the roof?
A somewhat related question is whether Bharat Biotech and India’s regulators can answer the disturbing questions raised by the Brazilian drug regulator’s report on Covaxin.
Will other foreign vaccines help?
The decision to allow other vaccines beyond SII’s Covishield and Covaxin, arbitrary as its timing may seem, should provide a boost to India’s vaccination campaign. The biggest player here is likely to be Russia’s Sputnik V, since the Russian Direct Investment Fund had already signed an agreement with five Indian producers to make 850 million doses a year.
Hyderabad-based Dr Reddy’s Laboratories has the rights to the first 100 million doses, and is likely to import the first lot. The company said that the first doses should arrive between now and June. Sputnik V was actually given approval before the government also opened up India’s campaign to any major foreign supplier (not including China’s Sinovac) on the same day.
Dr Reddy’s Laboratories was, in fact, conducting a bridging trial of the Russian vaccine, though no results have been declared. The vaccine has shown excellent efficacy numbers in studies, but has also run into trouble in Europe, with Slovakia for example claiming that the doses it received did not have “the same characteristics and properties” as the versions examined by researchers.
Initially it will need to be kept at extremely low temperatures – between minus 18°C to minus 22°C – until there is enough data for it to be approved in a “freeze-dried form”, which is one of the reasons few expect it to make a big dent to India’s August target, though it may play a large role in the second half of the year.
And that is before even getting to the cost question, which is yet to be hashed out with the government. Other foreign vaccines – Pfizer and Moderna – are likely to face similar challenges. The Johnson & Johnson vaccine, which will also be produced in India through a Quad arrangement, is set to begin its trials soon.
What about variants?
Maybe the scariest of these questions is, are the vaccines available in India useful against the variants that seem to be powering the second wave? An initial study has shown that the AstraZeneca vaccine, being manufactured by SII, is weaker against the South Africa variant.
We do not yet have data about the level of protection against the Indian variant, though Covaxin appears better suited than Covishield in this regard. At the moment, no vaccines that use the more adaptable mRNA method are available in India, though trials for one are beginning soon.
Thanks for reading the Political Fix. Send feedback to rohan@scroll.in.
Support our journalism by subscribing to Scroll+. We welcome your comments atletters@scroll.in.